Consequences of Chronic Cannabis Abuse
Disclaimer
This article is not complete yet. This is just a draft and its completion will take some time.
This article contains a collection of basic neurobiological knowledge. If you are interested in this topic, feel free to read the following sections. However, do not expect any conclusions.
The whole thing is kept very superficial. Gradually, information is being collected that links the following mentioned diseases to their respective neuropathological causes in order to get a general overview of the neurobiological functioning of cannabis abuse, withdrawal, and psychotropic drugs.
Preface
With over 24 million users, cannabis is the most widely used drug in the USA; a seemingly harmless substance if you smoke a joint in the morning - abuse, however should not be underestimated as it has many long term consequences.
Motivation
After 14 years of excessive THC consumption, the negative symptoms of drug abuse clearly outweigh its positive side effects. As part of a psychiatric treatment and withdrawal, I would like to understand on a neuro-biological level what effects years of abuse of various drugs have on the mammalian brain.
Mixed consumption of Nicotine, Cannabis, MDMA, Psylocybin and LSD is not harmless and can have devastating consequences.
I'm not trying to say drugs are bad (m'kay). I spent a lot of time enjoying my life. Excessive consume over years, however, is and always will be toxic to you, your body and your mental health.
Effects
The effects of years of drug abuse are devastating and range from confusion, memory disorders, excessive sweating, depression, anxiety, bipolarity, eating disorder and paranoia to panic attacks, dissociativity and schizoaffective disorder.
Treatments
The good part of the story is, you can fix all of this. It's a long way to go; it won't be easy, but it's indeed very possible to get back on track.
Side note: If you're being suicidal: Seek help. It helps. --- If you can't keep up living: Change your life; make it the one you want to live. --- Worldwide hotlines for suicide prevention --- Don't change the life of your loved ones, change yours! <3
Increased Risk of Developing Drug-Induced Psychotic Disorders in Patients with ADHD
ADHD
[ADHD] often correlates with substance abuse.[1] People who suffer from ADHD should be extra cautious dealing with drugs. Increased risk of addiction[[^adhd-substance-misuse-2]] and high prevalance[[^adhd-prevalance]] of comorbid disorders carry a high risk of developing psychotic and personality disorders.
Adolescents with attention-deficit/hyperactivity disorder (ADHD) are known to be at significantly greater risk for the development of substance use disorders (SUD) compared to peers. Impulsivity, which could lead to higher levels of drug use, is a known symptom of ADHD and likely accounts, in part, for this relationship. -- Childhood ADHD and Risk for Substance Dependence in Adulthood: A Longitudinal, Population-Based Study
To examine the 12-month prevalence, risk factors, and comorbidity of ADHD in a collective of adult psychiatric patients admitted to an open general ward in a psychiatric hospital in Schleswig-Holstein (Germany) over a period of one year
The 12-month prevalence of ADHD was 59.0 % (severe symptomatology: 33.1 %), high rates of comorbid disorders (92.9 % depression, 5.1 % bipolar disorder, 28.6 % anxiety disorder, 30.6 % emotional unstable (Borderline) personality disorder, 31.6 % avoidant personality disorder, 18.4 % dependent personality disorder, 25.5 % combined personality disorder, 10.2 % obsessive-compulsive personality disorder, 26.5 % PTSD, 25.5 % restless legs syndrome, 24.5 % adiposity, 11.2 % eating disorder, 45.9 % learning difficulty, 51.0 % ni cotine dependency, 4.1 % alcohol dependency, 7.1 % illegal substance dependency), risk factors for ADHD, a high genetic risk (72.4 %) and problems in psychosocial functioning. -- Attention-deficit/hyperactivity disorder (ADHD) in adult psychiatry: Data on 12-month prevalence, risk factors and comorbidity
Psychoses
Frequent long-term abuse of potent cannabis can cause psychotic symptoms and increase the risk of psychotic disorders such as schizophrenia or schizoaffective. [2, [^cannabis-schizo-evidence], [^cannabis-schizo-risk-value]]
Cannabis is a known risk factor for schizophrenia, although the exact neurobiological process through which the effects on psychosis occur is not well-understood.
Cannabis is involved in approximately 50% of psychosis, schizophrenia, and schizophreniform psychosis cases.[1,2,3,4,5] -- Cannabis and psychosis: Neurobiology
A neurobiological primer
I don't know anything about neurobiology yet. This article is just a primer; a compilation of basic neurobiological knowledge gathered from the internet. If you're interested in these topics, you probably already know most of what's being mentioned from here on.
! Spoiler: It's mostly Wikipedia.
Metabolism
THC, ADCY1, ATP, cAMP und die PKA Transkription
THC activates the CNR1, which inhibits ADCY1 in the synaptic cleft, leading to a reduction in ATP-cAMP transformation
All classes of adenylyl cyclase catalyse the conversion of adenosine triphosphate (ATP) to 3',5'-cyclic AMP (cAMP) and pyrophosphate.[4]
This results in chronic deficiency of the secondary messenger cAMP, and a reduction of PKA transcription, which is dependent on the cAMP concentration in the cell
PKA is also commonly known as cAMP-dependent protein kinase {...] as protein expression varies from cell type to cell type, the proteins that are available for phosphorylation will depend upon the cell in which PKA is present. Thus, the effects of PKA activation vary.
and may lead to a dystonia of PKA gene regulation in the Hippocampus, cerebellum and basal ganglia. Specifically affected are: PRKAR1A, PRKAR1B, PRKAR2A, PRKAR2B, PRKACA, PRKACB and PRKACG.
Basics
ATP - Adenosintriphosphat
Adenosine triphosphate (ATP) is a complex organic chemical that provides energy to drive many processes in living cells, e.g. muscle contraction, nerve impulse propagation, and chemical synthesis. Found in all forms of life, ATP is often referred to as the "molecular unit of currency" of intracellular energy transfer.[2] When consumed in metabolic processes, it converts either to adenosine diphosphate(ADP) or to adenosine monophosphate (AMP). Other processes regenerate ATP so that the human body recycles its own body weight equivalent in ATP each day.[3] It is also a precursor to DNA and RNA, and is used as a coenzyme.
From the perspective of biochemistry, ATP is classified as a nucleoside triphosphate, which indicates that it consists of three components: a nitrogenous base (adenine), the sugar ribose, and the triphosphate.
Wikipedia - Adenosine triphosphate
First Messenger - Ligand
In biochemistry and pharmacology, a ligand is a substance that forms a complex with a biomolecule to serve a biological purpose. In protein-ligand binding, the ligand is usually a molecule which produces a signal by binding to a site on a target protein. The binding typically results in a change of conformational isomerism (conformation) of the target protein. In DNA-ligand binding studies, the ligand can be a small molecule, ion,[1] or protein[2] which binds to the DNA double helix. The relationship between ligand and binding partner is a function of charge, hydrophobicity, and molecular structure. The instance of binding occurs over an infinitesimal range of time and space, so the rate constant is usually a very small number.
Binding occurs by intermolecular forces, such as ionic bonds, hydrogen bonds and Van der Waals forces. The association of docking is actually reversible through dissociation. Measurably irreversible covalent bonding between a ligand and target molecule is atypical in biological systems. In contrast to the definition of ligand in metalorganic and inorganic chemistry, in biochemistry it is ambiguous whether the ligand generally binds at a metal site, as is the case in hemoglobin. In general, the interpretation of ligand is contextual with regards to what sort of binding has been observed. The etymology stems from ligare, which means 'to bind'.
Ligand binding to a receptor protein alters the conformation by affecting the three-dimensional shape orientation. The conformation of a receptor protein composes the functional state. Ligands include substrates, inhibitors, activators, and neurotransmitters. The rate of binding is called affinity, and this measurement typifies a tendency or strength of the effect. Binding affinity is actualized not only by host–guest interactions, but also by solvent effects that can play a dominant, steric role which drives non-covalent binding in solution.[3] The solvent provides a chemical environment for the ligand and receptor to adapt, and thus accept or reject each other as partners.
Second Messenger
Second messengers are intracellular signaling molecules released by the cell in response to exposure to extracellular signaling molecules—the first messengers. (Intracellular signals, a non-local form or cell signaling, encompassing both first messengers and second messengers, are classified as juxtacrine, paracrine, and endocrine depending on the range of the signal.) Second messengers trigger physiological changes such as proliferation, differentiation, migration, survival, and apoptosis.
They are one of the triggers of intracellular signal transduction cascades.[1]
Examples of second messenger molecules include cyclic AMP, cyclic GMP, inositol trisphosphate, diacylglycerol, and calcium.[2] First messengers are extracellular factors, often hormones or neurotransmitters, such as epinephrine, growth hormone, and serotonin. Because peptide hormones and neurotransmitters typically are biochemically hydrophilicmolecules, these first messengers may not physically cross the phospholipid bilayer to initiate changes within the cell directly—unlike steroid hormones, which usually do. This functional limitation necessitates the cell to devise signal transduction mechanisms to transduce first messenger into second messengers, so that the extracellular signal may be propagated intracellularly. An important feature of the second messenger signaling system is that second messengers may be coupled downstream to multi-cyclic kinase cascades to greatly amplify the strength of the original first messenger signal[3][4]. For example, RasGTP signals link with the Mitogen Activated Protein Kinase (MAPK) cascade to amplify the allosteric activation of proliferative transcription factors such as Myc and CREB.
cAMP
Cyclic adenosine monophosphate (cAMP, cyclic AMP, or 3',5'-cyclic adenosine monophosphate) is a second messengerimportant in many biological processes. cAMP is a derivative of adenosine triphosphate (ATP) and used for intracellular signal transduction in many different organisms, conveying the cAMP-dependent pathway. It should not be confused with 5'-AMP-activated protein kinase (AMP-activated protein kinase).
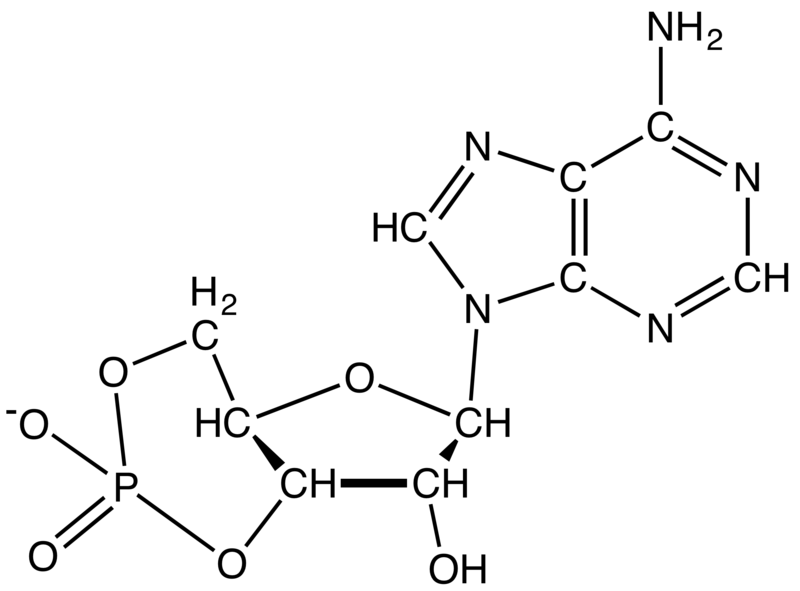
Wikipedia - Cyclic adenosine monophosphate
[...] It acts as an intracellular chemical messenger ( second messenger ) between the plasmalemma, where it is formed by adenylate cyclase from ATP ( adenosine triphosphate ) with elimination of pyrophosphate, and certain sites of the genome or certain enzyme systems of the plasma. The cAMP cascade is used for intracellular signal transduction , in which the incoming signal is additionally amplified ( see additional information ). CAMP- and calcium- dependent protein phosphorylation is more pronounced in the brainthan in any other organ. cAMP activates protein kinase A , which can phosphorylate many proteins. Various processes in the nervous system are cAMP-dependent, such as the regulation of myelinization of Schwann cells , the phosphorylation of the transcription factor CREB , the modulation of voltage-dependent ion channels or the expression of tyrosine hydroxylase . Cleavage of the 3 'phosphate ester moiety and thus inactivation of cAMP to adenosine 5'-monophosphate (AMP) is accomplished by a specific phosphodiesterase .
cAMP Cascade
The cAMP cascade is an intracellular signal transduction system in which the incoming signal is additionally amplified. The binding of a hormone to a cell receptor ( receptors ) starts the cascade. First, a G protein adjacent to the receptor is activated, which then binds a GTP (guanosine 5'-triphosphate) and cleaves its α subunit. This process induces the formation of cAMP until the GTP is hydrolyzed. The cAMP in turn activates protein kinase A, which catalyses another reaction. Until the goal of the cascade is reached, two more reaction steps may be necessary. The amplification of the signal is effected by each of the activated messengers inducing the next step until it is itself degraded or inactivated. The cAMP cascade is ubiquitous, but can trigger different metabolic processes in different cells.
Spektrum - cAMP (translated)
Brain Regions
Hippocampus
The hippocampus (from the Greek ἱππόκαμπος, "seahorse" from ἵππος hippos, "horse" and κάμπος kampos, "sea-monster") is a major component of the brain of humans and other vertebrates. Humans and other mammals have two hippocampi, one in each side of the brain. The hippocampus is part of the limbic system, and plays important roles in the consolidation of information from short-term memory to long-term memory, and in spatial memory that enables navigation. The hippocampus is located under the cerebral cortex in the allocortex,[1][2][3] and in primates it is in the medial temporal lobe. It contains two main interlocking parts: the hippocampus proper(also called Ammon's horn)[4] and the dentate gyrus.
In Alzheimer's disease (and other forms of dementia), the hippocampus is one of the first regions of the brain to suffer damage; short-term memory loss and disorientation are included among the early symptoms. Damage to the hippocampus can also result from oxygen starvation (hypoxia), encephalitis, or medial temporal lobe epilepsy. People with extensive, bilateral hippocampal damage may experience anterograde amnesia: the inability to form and retain new memories.
Since different neuronal cell types are neatly organized into layers in the hippocampus, it has frequently been used as a model systemfor studying neurophysiology. The form of neural plasticity known as long-term potentiation (LTP) was initially discovered to occur in the hippocampus and has often been studied in this structure. LTP is widely believed to be one of the main neural mechanisms by which memories are stored in the brain.
Cerebellum
Wikipedia - Cerebellum
The cerebellum (Latin for "little brain") is a major feature of the hindbrain of all vertebrates. Although usually smaller than the cerebrum, in some animals such as the mormyrid fishes it may be as large as or even larger.[1] In humans, the cerebellum plays an important role in motor control. It may also be involved in some cognitive functions such as attention and language as well as in regulating fear and pleasure responses,[2] but its movement-related functions are the most solidly established. The human cerebellum does not initiate movement, but contributes to coordination, precision, and accurate timing: it receives input from sensory systems of the spinal cord and from other parts of the brain, and integrates these inputs to fine-tune motor activity.[3] Cerebellar damage produces disorders in fine movement, equilibrium, posture, and motor learning in humans.[3]
Anatomically, the human cerebellum has the appearance of a separate structure attached to the bottom of the brain, tucked underneath the cerebral hemispheres. Its cortical surface is covered with finely spaced parallel grooves, in striking contrast to the broad irregular convolutions of the cerebral cortex. These parallel grooves conceal the fact that the cerebellar cortex is actually a continuous thin layer of tissue tightly folded in the style of an accordion. Within this thin layer are several types of neurons with a highly regular arrangement, the most important being Purkinje cells and granule cells. This complex neural organization gives rise to a massive signal-processing capability, but almost all of the output from the cerebellar cortex passes through a set of small deep nuclei lying in the white matterinterior of the cerebellum.[4]
In addition to its direct role in motor control, the cerebellum is necessary for several types of motor learning, most notably learning to adjust to changes in sensorimotor relationships. Several theoretical models have been developed to explain sensorimotor calibration in terms of synaptic plasticity within the cerebellum. These models derive from those formulated by David Marr and James Albus, based on the observation that each cerebellar Purkinje cell receives two dramatically different types of input: one comprises thousands of weak inputs from the parallel fibers of the granule cells; the other is an extremely strong input from a single climbing fiber.[5] The basic concept of the Marr–Albus theory is that the climbing fiber serves as a "teaching signal", which induces a long-lasting change in the strength of parallel fiber inputs. Observations of long-term depression in parallel fiber inputs have provided support for theories of this type, but their validity remains controversial.[6]
Receptors
CNR1
The Cannabinoid receptor type 1 (CB1), also known as cannabinoid receptor 1, is a G protein-coupled cannabinoid receptorthat in humans is encoded by the CNR1 gene.[5] The human CB1 receptor is expressed in the peripheral nervous systemand central nervous system.[5] It is activated by: endocannabinoids, a group of retrograde neurotransmitters that include anandamide and 2-arachidonoylglycerol (2-AG); plant phytocannabinoids, such as the compound THC which is an active ingredient of the psychoactive drug cannabis; and, synthetic analogs of THC. CB1 is antagonized by the phytocannabinoid tetrahydrocannabivarin (THCV).[6][7]
The primary endogenous agonist of the human CB1 receptor is anandamide.[5]
Wikipedia - G protein
G protein-coupled receptor
G protein-coupled receptors (GPCRs), also known as seven-(pass)-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptor, and G protein–linked receptors (GPLR), constitute a large protein family of receptorsthat detect molecules outside the cell and activate internal signal transduction pathways and, ultimately, cellular responses. Coupling with G proteins, they are called seven-transmembrane receptors because they pass through the cell membrane seven times.[2]
G protein-coupled receptors are found only in eukaryotes, including yeast, choanoflagellates,[3] and animals. The ligands that bind and activate these receptors include light-sensitive compounds, odors, pheromones, hormones, and neurotransmitters, and vary in size from small molecules to peptides to large proteins. G protein-coupled receptors are involved in many diseases, and are also the target of approximately 34% of all modern medicinal drugs.[4][5][6]
There are two principal signal transduction pathways involving the G protein-coupled receptors:
- the cAMP signal pathway and
- the phosphatidylinositol signal pathway.[7]
When a ligand binds to the GPCR it causes a conformational change in the GPCR, which allows it to act as a guanine nucleotide exchange factor (GEF). The GPCR can then activate an associated G protein by exchanging the GDP bound to the G protein for a GTP. The G protein's α subunit, together with the bound GTP, can then dissociate from the β and γ subunits to further affect intracellular signaling proteins or target functional proteins directly depending on the α subunit type (Gαs, Gαi/o, Gαq/11, Gα12/13).[8]
Wikipedia - G protein-coupled receptor
Enzymes
AC
Adenylyl cyclase (EC 4.6.1.1, also commonly known as adenyl cyclase and adenylate cyclase, abbreviated AC) is an enzymewith key regulatory roles in essentially all cells.[2] It is the most polyphyletic known enzyme: six distinct classes have been described, all catalyzing the same reaction but representing unrelated gene families with no known sequence or structural homology.[3] The best known class of adenylyl cyclases is class III or AC-III (Roman numerals are used for classes). AC-III occurs widely in eukaryotes and has important roles in many human tissues.[4]
All classes of adenylyl cyclase catalyse the conversion of adenosine triphosphate (ATP) to 3',5'-cyclic AMP (cAMP) and pyrophosphate.[4] Magnesium ions are generally required and appears to be closely involved in the enzymatic mechanism. The cAMP produced by AC then serves as a regulatory signal via specific cAMP-binding proteins, either transcription factors, enzymes (e.g., cAMP-dependent kinases), or ion transporters.
Wikipedia - Adenylyl cyclase)
Adenylate cyclase w, adenylate cyclase, abbr. AC, adenylyl c yclase , plasma membrane enzyme whose active site is on the cytosol side; catalyzes the conversion of ATP ( adenosine triphosphate ) into cyclic adenosine 3 ', 5'-monophosphate ( cAMP ). It is activated by G proteins after stimulation of membrane receptors (eg adenosine, adreno, dopamine, histamine, opiate receptors) (by G s proteins) or inhibited (G i proteins). AC type I is also activated by calmodulin and intracellular calcium . This signal transduction transforms an extracellular signal ( hormones , growth factors and other regulatory molecules ) into an intracellular cAMP signal ( secondary messengers ). Since adenylate cyclase on a stimulus several cAMP molecules are formed, there is an amplification of the signal. Adenylate cyclase is antagonized by a specific phosphodiesterase that hydrolyzes cAMP to adenosine 5'-monophosphate. By resulting from this equilibrium cAMP level enzyme systems and the expression of certain genes are regulated. The activity of AC can be habituated or sensitized via receptor kinists and antagonists via protein kinases and transcriptionally regulated. - Inhibition of AC leads to memory and learning defects. Activity changes in AC are involved in the development of drug addiction ( addiction ) (inhibition of adenylate cyclase by binding to the opiate receptor ). Adenylate cyclase is also a target site for toxins: The mechanisms of action of cholera toxin and pertussis toxin are based on a permanent activation of adenylate cyclase. adrenergic receptors .
Spektrum - Adenylate cyclase (translated)
PKA
In cell biology, protein kinase A (PKA[N 1]) is a family of enzymes whose activity is dependent on cellular levels of cyclic AMP (cAMP). PKA is also known as cAMP-dependent protein kinase (EC 2.7.11.11). Protein kinase A has several functions in the cell, including regulation of glycogen, sugar, and lipid metabolism.
Wikipedia - Protein kinase A
PKA plays a central role in nervous signal transduction you might want to read the whole article especially the sections Activation and Function
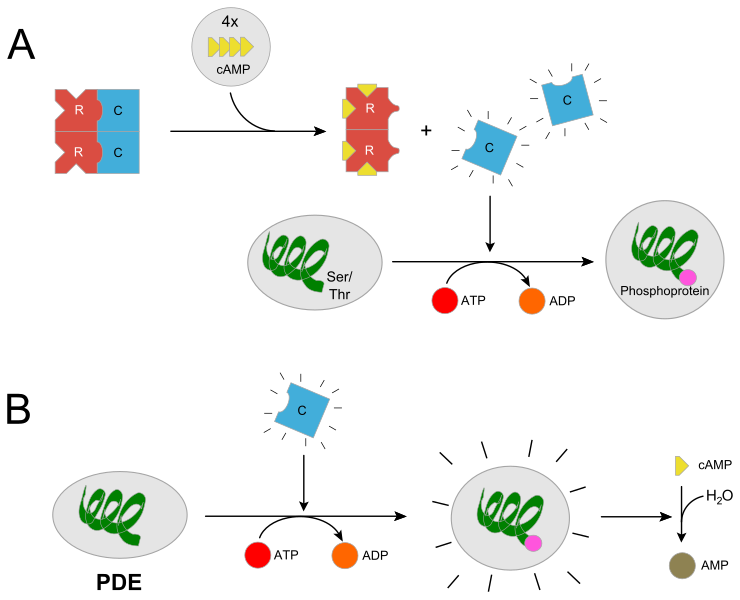
Proteins
G-Protein
G proteins, also known as guanine nucleotide-binding proteins, are a family of proteins that act as molecular switches inside cells, and are involved in transmitting signals from a variety of stimuli outside a cell to its interior. Their activity is regulated by factors that control their ability to bind to and hydrolyze guanosine triphosphate (GTP) to guanosine diphosphate (GDP). When they are bound to GTP, they are 'on', and, when they are bound to GDP, they are 'off'. G proteins belong to the larger group of enzymes called GTPases.
There are two classes of G proteins. The first function as monomeric small GTPases (small G-proteins), while the second function as heterotrimeric G protein complexes. The latter class of complexes is made up of alpha (α), beta (β) and gamma (γ) subunits.[1] In addition, the beta and gamma subunits can form a stable dimeric complex referred to as the beta-gamma complex [2].
Heterotrimeric G proteins located within the cell are activated by G protein-coupled receptors (GPCRs) that span the cell membrane.[3]Signaling molecules bind to a domain of the GPCR located outside the cell, and an intracellular GPCR domain then in turn activates a particular G protein. Some active-state GPCRs have also been shown to be "pre-coupled" with G proteins.[4] The G protein activates a cascade of further signaling events that finally results in a change in cell function. G protein-coupled receptor and G proteins working together transmit signals from many hormones, neurotransmitters, and other signaling factors.[5] G proteins regulate metabolic enzymes, ion channels, transporter proteins, and other parts of the cell machinery, controlling transcription, motility, contractility, and secretion, which in turn regulate diverse systemic functions such as embryonic development, learning and memory, and homeostasis.[6]
Wikipedia - G protein
CREB
CREB-TF (CREB, cAMP response element-binding protein)[1] is a cellular transcription factor. It binds to certain DNA sequences called cAMP response elements (CRE), thereby increasing or decreasing the transcription of the genes.[2] CREB was first described in 1987 as a cAMP-responsive transcription factor regulating the somatostatingene.[3]
Genes whose transcription is regulated by CREB include: c-fos, BDNF, tyrosine hydroxylase, numerous neuropeptides (such as somatostatin, enkephalin, VGF, corticotropin-releasing hormone),[2] and genes involved in the mammalian circadian clock (PER1, PER2).[4]
CREB is closely related in structure and function to CREM (cAMP response element modulator) and ATF-1 (activating transcription factor-1) proteins. CREB proteins are expressed in many animals, including humans.
CREB has a well-documented role in neuronal plasticity and long-term memory formation in the brain and has been shown to be integral in the formation of spatial memory.[5] CREB downregulation is implicated in the pathology of Alzheimer's disease and increasing the expression of CREB is being considered as a possible therapeutic target for Alzheimer's disease.[6] CREB also has a role in photoentrainment in mammals.
Wikpedia - CREB
CREM
cAMP responsive element modulator is a protein that in humans is encoded by the CREM gene,[5][6][7] and it belongs to the cAMP-responsive element binding protein family. It has multiple isoforms, which act either as repressors or activators.[8]CREB family is important for in regulating transcription in response to various stresses, metabolic and developmental signals.[9] CREM transcription factors also play an important role in many physiological systems, such as cardiac function,[10] circadian rhythms,[11] locomotion and spermatogenesis
Enzymes / Genes
ADCY1
Adenylyl cyclase type 1 is an enzyme that in humans is encoded by the ADCY1 gene.[5][6]. This gene encodes a form of adenylyl cyclase expressed in brain
ADCY1 is a calmodulin-sensitive adenylyl cyclase. In terms of function, It may be involved in regulatory processes in the central nervous system; specifically, it may play a role in memory acquisition and learning. It is inhibited by the G proteinbeta and gamma subunit complex.[7]
Wikipedia - ADCY1
This gene encodes a member of the of adenylate cyclase gene family that is primarily expressed in the brain. This protein is regulated by calcium/calmodulin concentration and may be involved in brain development. Alternate splicing results in multiple transcript variants.
ADCY1 (Adenylate Cyclase 1) is a Protein Coding gene. Diseases associated with ADCY1 include Deafness, Autosomal Recessive 44 and Autosomal Recessive Non-Syndromic Sensorineural Deafness Type Dfnb. Among its related pathways are RET signaling and Oocyte meiosis. Gene Ontology (GO) annotations related to this gene include nucleotide binding and phosphorus-oxygen lyase activity. An important paralog of this gene is ADCY5.
Genecards - [ADCY1]:gene:ADCY1
Amino acids
Tyrosin
Tyrosin (abgekürzt Tyr oder Y) ist in seiner natürlichen L-Form eine nichtessentielle proteinogene α-Aminosäure, die in den meisten Proteinen vorkommt. Tyrosin ist Ausgangssubstanz für die Biosynthese von DOPA, Dopamin, Katecholaminen, Melanin, Thyroxin und Tyramin. Die Biosynthese erfolgt in vielen Tieren aus der essentiellen Aminosäure Phenylalanin, eine Beeinträchtigung dieses Weges kann vielfältige Defekte auslösen.
Wenn in diesem Text oder in der wissenschaftlichen Literatur „Tyrosin“ ohne weiteren Namenszusatz (Präfix) erwähnt wird, ist L-Tyrosin gemeint.
Signaling Pathways
Cannabinoid receptor signaling (homo sapiens)
The endocannabinoid system (ECS) is a biological system composed of endocannabinoids, which are endogenous lipid-based retrograde neurotransmitters that bind to cannabinoid receptors, and cannabinoid receptor proteins that are expressed throughout the vertebrate central nervous system (including the brain) and peripheral nervous system. The endocannabinoid system is involved in regulating a variety of physiological and cognitive processes including fertility,[1] pregnancy,[2]during pre- and postnatal development,[3] appetite, pain-sensation, mood, and memory, and in mediating the pharmacological effects of cannabis.[4][5] The ECS is also involved in mediating some of the physiological and cognitive effects of voluntary physical exercise in humans and other animals, such as contributing to exercise-induced euphoria as well as modulating locomotor activity and motivational salience for rewards.[6][7][8][9] In humans, the plasma concentration of certain endocannabinoids (i.e., anandamide) have been found to rise during physical activity;[6][7] since endocannabinoids can effectively penetrate the blood–brain barrier, it has been suggested that anandamide, along with other euphoriant neurochemicals, contributes to the development of exercise-induced euphoria in humans, a state colloquially referred to as a runner's high.[6][7]
Two primary endocannabinoid receptors have been identified: CB1, first cloned in 1990; and CB2, cloned in 1993. CB1 receptors are found predominantly in the brain and nervous system, as well as in peripheral organs and tissues, and are the main molecular target of the endocannabinoid ligand (binding molecule), anandamide, as well as its mimetic phytocannabinoid, THC. One other main endocannabinoid is 2-arachidonoylglycerol (2-AG) which is active at both cannabinoid receptors, along with its own mimetic phytocannabinoid, CBD. 2-AG and CBD are involved in the regulation of appetite, immune system functions and pain management.[10][11][12]
Dopamine Metabolism
Dopamine (DA, a contraction of 3,4-dihydroxyphenethylamine) is an organic chemical of the catecholamine and phenethylamine families. It functions both as a hormone and a neurotransmitter, and plays several important roles in the brain and body. It is an amine synthesized by removing a carboxyl group from a molecule of its precursor chemical L-DOPA, which is synthesized in the brain and kidneys. Dopamine is also synthesized in plants and most animals. In the brain, dopamine functions as a neurotransmitter—a chemical released by neurons (nerve cells) to send signals to other nerve cells. The brain includes several distinct dopamine pathways, one of which plays a major role in the motivational component of reward-motivated behavior. The anticipation of most types of rewards increases the level of dopamine in the brain,[2][not in citation given] and many addictive drugs increase dopamine release or block its reuptake into neurons following release. Other brain dopamine pathways are involved in motor control and in controlling the release of various hormones. These pathways and cell groups form a dopamine system which is neuromodulatory.
Mechanisms
Gene regulation
Regulation of gene expression, or gene regulation,[1] includes a wide range of mechanisms that are used by cells to increase or decrease the production of specific gene products (protein or RNA). Sophisticated programs of gene expression are widely observed in biology, for example to trigger developmental pathways, respond to environmental stimuli, or adapt to new food sources. Virtually any step of gene expression can be modulated, from transcriptional initiation, to RNA processing, and to the post-translational modification of a protein. Often, one gene regulator controls another, and so on, in a gene regulatory network.
Gene regulation is essential for viruses, prokaryotes and eukaryotes as it increases the versatility and adaptability of an organism by allowing the cell to express protein when needed. Although as early as 1951, Barbara McClintock showed interaction between two genetic loci, Activator (Ac) and Dissociator (Ds), in the color formation of maize seeds, the first discovery of a gene regulation system is widely considered to be the identification in 1961 of the lac operon, discovered by François Jacob and Jacques Monod, in which some enzymes involved in lactose metabolism are expressed by E. coli only in the presence of lactose and absence of glucose.
In multicellular organisms, gene regulation drives cellular differentiation and morphogenesis in the embryo, leading to the creation of different cell types that possess different gene expression profiles from the same genome sequence. Although this does not explain how gene regulation originated, evolutionary biologists include it as a partial explanation of how evolution works at a molecular level, and it is central to the science of evolutionary developmental biology ("evo-devo").
The initiating event leading to a change in gene expression includes activation or deactivation of receptors.
Articles
Here's a list of articles worth reading.
PMID: 27663939 [^adhd-substance-misuse-2]:Childhood ADHD and Risk for Substance Dependence in Adulthood: A Longitudinal, Population-Based Study PMID: 25162629 | PMCID: PMC4146503 [^adhd-dopamine]: Attention-deficit-hyperactivity disorder and reward deficiency syndrome - PMID: 19183781 | PMCID: PMC2626918 [^adhd-prevalance]:Attention-deficit/hyperactivity disorder (ADHD) in adult psychiatry: Data on 12-month prevalence, risk factors and comorbidity PMID: 29490380
PMID: 18560513 | PMCID: PMC2424288 [^cannabis-schizo-evidence]: Cannabis and psychosis: Neurobiology PMID:29490380 [^cannabis-schizo-risk-value]:Cannabis consumption and psychosis or schizophrenia development PMID:30442059

